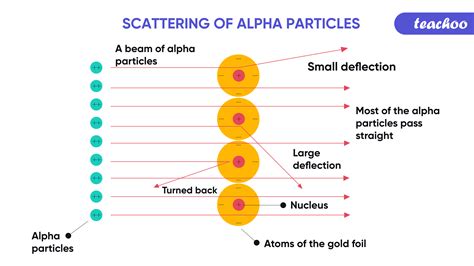
In 1911, Ernest Rutherford, along with his assistants H. Geiger and E. Marsden, conducted a groundbreaking experiment that revolutionized our understanding of the atomic structure. The experiment involved shooting alpha particles at a thin gold foil and observing how they were deflected or scattered by the atoms in the foil. This pioneering work led to the development of Rutherford's nuclear model of an atom, which fundamentally changed the way we understand the structure of matter.
The Experiment
To conduct this experiment, Rutherford and his team prepared a very thin gold foil, with a thickness of about 10^-4 cm. They then directed a beam of alpha particles, emitted by radioactive polonium nuclei, at the gold foil in a precise narrow line perpendicular to the foil. The alpha particles were positively charged and fairly massive, making them an ideal probe for studying the atomic structure.
The Results
The result of the experiment was initially counter-intuitive. Most of the alpha particles passed through the gold foil undeflected, as if there had been nothing in their path! A smaller number of the particles were deflected sharply as they passed through the foil, and a very small fraction of the alpha particles were reflected backwards off of the gold foil.
Interpretation
How can we simultaneously account for the lack of any deflection for most of the alpha particles and for the deflection through large angles of a very small number of particles? Rutherford's team concluded that most of the volume of each gold atom is empty space, containing nothing which might deflect an alpha particle. This led them to propose that all of the positive charge and most of the mass of an atom is contained in a nucleus.
The Nuclear Model
A detailed calculation based on this model revealed that the nucleus must be about 100,000 times smaller than the size of the atom itself. The electrons, already known to be contained in the atom, must be outside of the nucleus, since the nucleus is positively charged. They must move in the remaining space of the much larger volume of the atom. Moreover, in total, the electrons comprise less than 0.05% of the total mass of an atom.
Rutherford's nuclear model of an atom describes it as an electrically neutral sphere with a small and positively charged nucleus at the centre, surrounded by revolving electrons in their dynamically stable orbits. The centripetal force that keeps the electrons in their orbits is an outcome of the electrostatic force of attraction between the positively charged nucleus and the negatively charged revolving electrons.
References
- Rutherford, E. (1911). Scattering of Alpha Particles by Atoms.
- Geiger, H., & Marsden, E. (1911). Scattering of Alpha Particles by Atoms.
Solved Example for You
Question: Rutherford, Geiger and Marsden, directed a beam of alpha particles on a foil of which metal?
A) Platinum
B) Tungsten
C) Gold
D) Silver
Solution: C) Gold