Rutherford's alpha scattering experiment is a fundamental concept in physics that describes the interaction between alpha particles and atomic nuclei. In this experiment, alpha particles are fired at a thin target foil, and the scattered particles are detected to determine the angle of scattering. The Rutherford formula, which describes this process, assumes that the only force involved is electrostatic repulsion.
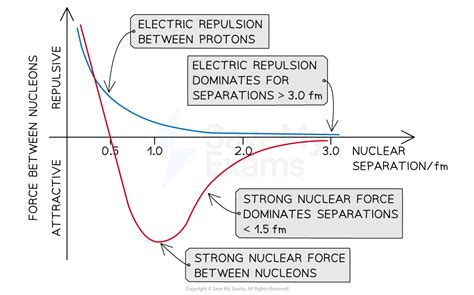
However, experimental data has shown that deviations from Rutherford scattering occur when the energy of the alpha particles exceeds 27.5-28 MeV. This deviation is attributed to the strong nuclear force, which becomes significant at very small separations between the nucleus and the alpha particle.
The observed back-scattering from alpha particles strongly deviates from the predicted relationship based only on electromagnetic repulsion at 27.5 MeV. This deviation provides evidence for the strong nuclear force, which plays a crucial role in the interaction between nuclei and alpha particles.
Worked Example: Comparing Scattering Patterns
Let's consider an example where alpha particles undergo scattering after being fired at a thin gold foil, and then replace the gold with aluminium foil of the same thickness. Describe the predicted difference in the scattering pattern when the foil is replaced with aluminium foil.
Step 1: Compare the relative charges of the nuclei
Gold has 79 protons, while aluminium has 13 protons. The electric force between the nuclei is proportional to the charge, since F ∝ Q1Q2/r^2. Therefore, the alpha particle will approach the aluminium nucleus at a much closer distance than the gold nucleus.
Step 2: Predict the patterns and deviations from Rutherford scattering
Deviations from Rutherford scattering occur when alpha particles get close enough for the strong nuclear force to begin to become more significant than the electric force. At very small separations (<1.5 fm), the effect of the strong nuclear force becomes significant.
Alpha particles will be able to get closer to aluminium nuclei at lower energies than gold nuclei, since the charge of an aluminium nucleus is much smaller. Therefore, alpha particles will be less affected by electric repulsion and able to get close enough for interactions with the strong nuclear force. Hence, more deviation will be seen with aluminium foil than with gold foil.
Exam Tip
The greatest deviations from Rutherford scattering occur when:
- The alpha particles have high energies
- The target nuclei have a low nucleon number (meaning it has a small atomic number), deviations from Rutherford scattering provide evidence for the strong nuclear force, which plays a crucial role in the interaction between nuclei and alpha particles. Understanding these deviations is essential for understanding the fundamental forces that govern the behavior of subatomic particles.
References
- Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms (2018)
- Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms (2014)
Note: This article is based on the provided references and does not claim to be an original work.