Flow cytometry is a powerful tool for analyzing cells based on their physical and chemical properties. One of the most important aspects of flow cytometry is gating, which involves identifying specific cell populations based on their characteristics. In this article, we will discuss various gating strategies that can be used to identify cells of interest, exclude debris, and analyze samples with multiple cell types.
Forward vs Side Scatter (FSC vs SSC) Gating
FSC vs SSC gating is commonly used to identify cells of interest based on size and granularity (complexity). It is often suggested that forward scatter indicates cell size, while side scatter relates to the complexity or granularity of the cell. However, it should be noted that forward scatter does not necessarily relate to size, and side scatter is not really granularity. These are indications based on light refraction, which depend on the sample, sheath fluid, and laser wavelength.
For example, FSC vs SSC gating is most useful for blood samples, but even then, granulocytes (12-17 μm) can sometimes appear larger than monocytes (20-25 μm). Furthermore, for small samples, the level of light scatter does not always correlate with size. Despite these caveats, this first-level gating method is useful for identifying cells of interest.
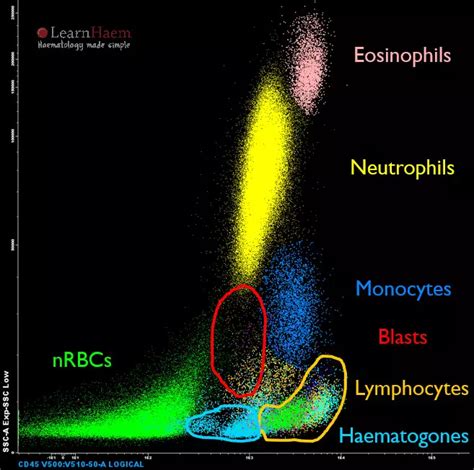
Figure 1 shows how FSC vs SSC gating can be used to identify distinct cell types in red cell lysed whole blood.
Excluding Debris
Debris tends to have lower forward scatter levels and can often be found at the bottom left corner of the FSC vs SSC density plot (Figure 2). Gating on FSC vs SSC can be used to exclude debris from the analysis.
Doublet Exclusion
Doublet cells can significantly affect your analysis and could lead to inaccurates. A forward scatter height (FSC-H) vs. forward scatter area (FSC-A) density plot can be used to exclude doublets as shown in Figure 3 below. A side scatter height (SSC-H) vs side scatter area (SSC-A) plot can also be used.
Single Parameter Histograms
Single parameter histograms can be used to further identify distinct cell types that express a particular marker in a specific population of cells. For example, after gating for lymphocytes on red cell lysed whole blood (Figure 1), a CD3 single parameter histogram can be generated to identify CD3-expressing cells (Figure 4).
Two-Parameter Density Plots
Two-parameter density plots, in which each axis represents a particular marker that your samples have been stained for, can be used to further analyze your samples. For example, after gating on a lymphocyte gate on whole blood as in Figure 1, a two-parameter density plot can be used to distinguish T cells and B cells by creating a plot on CD3 vs CD19 (Figure 5).
Backgating
Backgating is a common method for confirming a staining pattern or gating method. This can be useful for getting additional information to identify your cells if you are unsure of your gates, the expression levels or your cells, or whether dead cells have been included in your analysis.
By playing around with the data and checking everything, you can now confidently analyze your cells and make accurates based on your flow cytometry analysis.
Lerning More
To learn more about gating and see a full-length gating example, please visit our website.